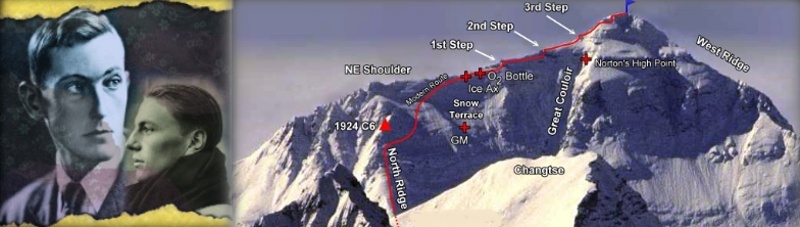
Left: George Mallory and Andrew Irvine © RGS/The Sandy Irvine Trust, from "Ghosts of Everest" ; Right: 1924 North Face locations © Pete Poston
| Photoanalysis | Routes & Maps | Video & Books | Contact Me |


"I'm quite doubtful if I shall be fit enough. But again I wonder if the monsoon will give us a chance. I don't want to get caught, but our three-day scheme from the Chang La will give the monsoon a good chance. We shall be going up again the day after tomorrow. Six days to the top from this camp!"
--from George Mallory's last letter to his wife prior to disappearing on Mt. Everest with his partner Andrew "Sandy" Irvine in 1924
"My face is in perfect agony. Have prepared two oxygen apparatus for our start tomorrow morning".
- Sandy Irvine's last diary entry

Why the Camera and Film are Not Doomed to
Destruction!
by Pete Poston © 2011
Note: I still have more to add! - e.g. the tin cans in the 20's were made from "tin plate", or tin-coated steel, and the effect of the black paint on the camera under solar illumination.
Background
On the internet there are claims that because of the (supposedly) significant amounts of moisture and rusting on the North Face of Mount Everest, there is no hope that the vulnerable camera and film could have survived intact (Figure 1, ref. 1).

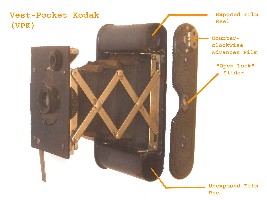
Figure 1: The VPK Kodak camera, left © William Yowell, right © Tom Holzel
Citing all the rusting that has occurred to the artifacts found on Mallory and elsewhere (exaggerated as we will see), it is claimed that the camera has certainly rusted open and the precious film destroyed. Any who search for Irvine and the camera are foolish and risking lives.
All this is well and good – for years researchers have understood there is a risk the camera and film might have been destroyed.
So what? What’s so “new” about this?
The Artifacts
A number of years ago I was able to visit an exhibition of Mallory and Irvine’s artifacts at the Washington State Historical Society in Tacoma, WA. So I dug out my old pictures and examined them for the extent of rusting. The photos in Figure 2 show the oxygen bottle, and artifacts from the 1924 and 1933 high camps.
Click a thumbnail for a higher resolution image –
(d) |
(e) |
Figure 2: Artifacts from the 1924 and 1933 high camps (all photos taken by the author)
(a) artifacts from M&I’s Camp 6,
including the mitten found on the NE Ridge
(b) close up of these artifacts.
(c) artifacts from the 1933 camp closest to Irvine’s presumed location.
(d) close up of perfectly preserved artifacts from the 1933 C 6.
(e) the oxygen bottle found at the base of the 1st Step.
There’s pitifully little in the way of artifacts from the 1924 high camp. Most of the metal was oxidized, there’s no doubt. The question is, did the rusting eat completely through? At least Norton’s smelly sock made it through the decades in good condition, along with a silver spoon (of course). A piece of paper from a tea box also miraculously survived tucked away in a crack - still readable, but not really decayed.
Some of Mallory’s possessions on his body are rustier, in particular see the scissors in Figure 3. There is a possible electrochemical reason for this; dissimilar metals in contact with each other can react in what is called an oxidation-reduction reaction (the same happens when iron rusts). I don’t know what other metal items were in the same pocket or pouch, however.
The notable exception is the near-perfect condition of Mallory’s watch, which started ticking after lying frozen in Mallory’s pocket for 75 years. Why didn’t all this rusting destroy the delicate watch mechanism?


Figure 3: left: Mallory’s rusty scissors (© Jochen Hemmleb), right: Mallory’s watch (© Rick Reanier/Jochen Hemmleb)
The oxygen bottle was also found in the vicinity of where Irvine is thought to lie. Rusting, yes – but really insignificant. And compared to the North Col photo, is there really much of a difference?
But the artifacts found in the 1933 high camp, near Irvine’s probable resting place at 8400 meters, are in excellent condition. Take a look at the close-up in Figure 2(b) – you’ll find very little oxidation. You’ll also want to go to Jake Norton’s blog and look at how incredibly well-preserved (and tasty-looking) the cans of spaghetti and beans look (I’m hungry while writing this).
Check out the paper wrappings on some of the old cans in Figure 2(d) – they are also nearly perfectly preserved. Where’s all the water damage? If this is the extent of damage to the paper covers, then how much damage could have occurred to the camera?
Explanations
(a) Is the camera really all that vulnerable to moisture and rusting?
Even if the camera has rusted open or simply been damaged in a fall, is the film doomed to destruction?
There are certainly significant reasons for concern. Tom Holzel has posted on his webpage a letter from a Kodak expert explaining the potential problems and solutions (there’s been some tweaking over the years). And the services of these very experts, while they are retired, are available when needed (ref. 2).
The experts tell us that the 1924 film is susceptible to cosmic rays, which can lead to enough clouding to ruin any image. There are also issues with the brittleness of old cellulose nitrate-base film, and “core-set” on supply and take-up spools that could ruin the entire roll.
Fortunately there are darkroom techniques that can help minimize some of these effects. If the film emulsion is brittle, a pre-hardening reagent can be added. And since the development process can lead to fogging, Kodak Antifog should be used.
What other mitigating circumstances are there? The camera was provided with a leather case that would help. And of course the deep freeze the film has been in for the last 85-90 years wouldn’t have hurt, either.
Another consideration is that while the film spool itself is made of metal, the exposed film is coiled up with the emulsion protected by a lining of paper. So the paper and would help protect the film, even with the camera busted open.
(b) Would the camera rust shut instead?
Bill Yowell is a long-time Mallory and Irvine researcher who had a great idea – isn’t it more likely that the camera would rust shut instead? The locking mechanism is quite tight – he knows because he owns one. Once the camera had rusted shut, it would be even more impervious to moisture.
Secondly, if Mallory and Irvine were anticipating using the Autographic feature of the VPK, the backing would not be paper but plastic. When scratched by the stylus the backing would become transparent, allowing a brief exposure of sunlight to record the image of the writing on the space between frames.
(c) Exposure to the elements
After examining the artifacts found on Mallory at the 1924 high camp (26,700’), and at the 1933 high camp (27,300’), I’m going to make the argument that the artifacts found at the higher altitude are actually in better condition than those lower down.
There’s a reason for this difference – the degree of exposure to the elements.
Mallory’s body and his high camp were totally exposed to the screaming wind and weather that sweeps across the North Ridge. When the camp was passed by in 1933 it was already completely ripped open.
The tent must have been buried in a rock fall at some point, too, because when discovered in 2001, the contents of the tent had spilled its guts down the steep slope below. To emphasize the point, prominently displayed at the Washington State Historical Society exhibition were the broken and splintered remains of their tent poles – see Figure 2(a).
The remains of the 1933 tent, however, covered and protected the artifacts, and so they were less exposed to the hostile weather conditions. And look at the pristine condition of the leather on the porter’s pack-frame in the upper left part of Figure 2(c). Again, given that the camera is kept in a leather case, this bodes well for the preservation of the film.
(d) Microorganisms
I also wonder if there are any cold-tolerant micro-organisms that could be responsible for some of the rusting (ref. 3 - 5). These types of bacteria are known as psychrophiles (“cold-loving” bacteria) and have been found in deep sea, cold-water environments and on the Rongbuk Glacier. There are both reducing (anaerobic) and oxidizing forms of psychrophiles, some that use iron as part of their metabolism. Are there psychrophiles at higher altitudes? Could they be responsible for some, or even most of the rusting?
It’s an interesting research project, and if any readers have further information, please contact me.
(e) The difference between relative humidity and the actual amount of water
Relative humidity is the ratio of actual water content to the maximum amount air can hold at a given temperature. What about the actual water content at high altitude? It’s much less under conditions of high altitude/low pressure as compared to sea level, even at the same relative humidity.
At the summit of Mount Everest, where the atmospheric pressure is about one-third that at sea level and the temperature is -20 oC (ref. 6), solid water will sublimate instead of evaporate as has been well understood on Everest since the 1920’s.
OK, calculation time (this is where your eyes will glaze over; see the Appendix for these general chemistry-style calculations).
What I want to do is calculate how much iron could be consumed by the oxygen inside the camera. I’ll do this calculation under monsoon conditions when the relative humidity is at its maximum. The data I have are from the summit, which is close enough to Irvine’s presumed location at 8400 meters.
The steps of the calculation are -
1. calculate the volume of water in the camera – this will be the maximum amount since the camera is not empty.
2. calculate the pressure of oxygen in the camera – this will determine its water solubility
3. determine the amount of dissolved oxygen – this assumes that the total water content is in one volume, not vaporized.
4. calculate the maximum amount of iron that could be consumed by this amount of dissolved oxygen
So here are the results:
· Under summit conditions the maximum amount of water that air can hold is 0.00095 atm (found in standard tables).
· During the monsoon the relative humidity at the summit is 70%, and given the maximum vapor pressure is 0.00095 atm, then the actual vapor pressure of water is 0.00067 atm.
· When you do the calculation based on this, the maximum volume of water in the camera is 74 microliters (a microliter is one-millionth of a liter).
· How much oxygen is present in the camera (remember that this is an empty camera)? The calculation results in about 13 milligrams of oxygen.
· Not all of this oxygen reacts however, because in the rusting process oxygen has to be dissolved in the water.
· So when you calculate the mass of oxygen in the water, it’s equal to about 1-microgram (one-millionth of a gram).
· Based on these results, a simple calculation reveals that about 2-micrograms (2-millionths of a gram) of iron will be consumed.
Not a heck of a lot, but how long would this take?
A rule of thumb is that the rate of a reaction decreases by a factor of two for every 10 oC drop in temperature. At high altitude where the vapor pressure of ice is so low, the rate of this reaction slows down by a factor of 2 x 2 x 2 x 2 = 16 times slower when the temperature drops from 20 oC to -20 oC.
What this translates into is over the 75-80 years that the artifacts were on Everest, the same amount of rusting would have occurred in about 5 years at sea level. Not a lot of time to eat through the camera leather, metal spool, backing paper or plastic, and finally the film.
The results of these calculations demonstrate that not only is there very little water vapor at extreme altitudes, but the rate of reaction is very slow and the amount of iron consumed is miniscule.
(f) The Monsoon
What about the effect of a thick layer of monsoon snow - corresponding to an increase in pressure to 10 atm, for example? (see the water phase diagram in Figure 4). Could liquid water be “squeezed” out of the snow and ice?
Normally, increasing the pressure on a substance makes it denser, perhaps undergoing changes in crystal structure as the pressure increases.
Water is weird this way, though - increasing pressure actually turns it into a liquid! This is why figure skaters don’t fall flat on their faces – the pressure of the skate produces a lubricating layer of liquid water to skoot around on.
So it’s conceivable that under the snowpack, some liquid water could have formed near Mallory’s body. There’s an important caveat, however – the temperature under the snowpack must be very close to freezing (again, see Figure 4). Otherwise the liquid-solid phase transition is quickly reached and water can exist only as a solid.
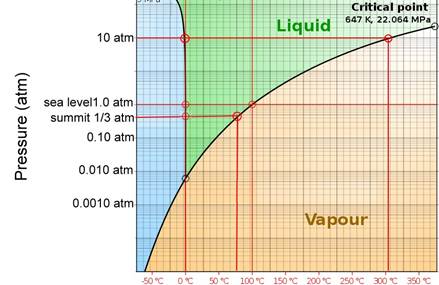
Figure 4: Phase diagram of water (Adapted from Wikipedia).
It seems to me that upwelling monsoon clouds would be a far more probable source of moisture as it wells up from lower elevations. I have been to the North side of Everest at the end of the monsoon, and watched these clouds roll in from the west. I have seen the same upwelling of moisture-laden clouds from the top of Gokyo Ri on the south side as well, so I know where they go.


Left: Deep
monsoon snow on the North Face,
Right: Sunset from Gokyo Ri
(© Pete Poston)
But would the moisture in the monsoon clouds reach Mallory or Irvine’s resting places? Most certainly not – the other thing I saw at Rongbuk was a very, very thick layer of monsoon snow.
On a similar note, storm clouds and high winds are the probable reason why verglas was seen in the Yellow Band by climber Frank Smythe in 1933 (this was not during the monsoon). It must be an extremely rare event since modern climbers when asked don’t recall seeing any at all.
(g) Supercooled water
There are circumstances in which a liquid will cool below its freezing point, but still remain in the liquid state. These fluids are what is referred to as “supercooled”, and are known to exist underneath glaciers, for example. It’s possible this state of water could exist on Everest, and so there would be liquid water even when the temperature drops to around -20 oC.
However, in order to reach a super-cooled state, the liquid must be super-pure (my word), that is, no dissolved substances such as ions. Otherwise, the dissolved substances will furnish nucleation centers, and solid water will crystallize out around these centers.
So the question is - could super-cooled water exist at high altitude on Everest? The odds seem pretty low because, (1) liquid water would have to form first, (2) we’re talking about snowfall at high elevation, not a glacier, and (3) the snowfall in the Everest region is known to contain dissolved ions such as chloride, as found in ice cores (Ref. 7). The origin of the chlorine is from seawater, and so during the monsoon this chlorine-rich water blows into the Everest region.
Is there super-cooling high up on Everest? I doubt it.
What Everest climbers and Arctic explorers tell us
Let’s forget all the theorizing - why not ask a climber who was actually there? A case in point is the discovery by Jake Norton in 2001 (ref. 8) of unspoiled foodstuffs in the 1933 British C 6 at 8300 m in the Yellow Band. I’ve already told you about the can of beans.
In this camp, a hungry Jake found biscuits in a state of perfect preservation. After eating a few, Norton found them to be a tad stale but completely palatable (he admits to being quite hungry - good thing he didn’t have a can opener).
Surely any exposure to enough moisture to cause extensive rusting (by that I mean it penetrated into the camera) would have certainly resulted in signs of decay of the biscuits, but none is observed.
There’s more evidence that’s been ignored – here is what Ralph Wondraschek had to say on Jake’s blog:
“Regarding the possibility of the Mallory/Irvine film emulsion having been preserved intact: why not look at another historical occurrence……the successful retrieval, and development procedure of the film emulsion of the Andrée, Strindberg and Fraenkel expedition.
The film was stored in Arctic weather conditions (with a relative high percentage of moisture) from 1897 to 1933 (when the three dead bodies were discovered on Kvitö Island), yet still delivered perfect pictures after its successful development after such a long time.
Would you care to explain why the case of the Mallory/Irvine film would be different? [emphasis added]
And no, this old emulsion is NOT sensitive to cosmic radiation, so no problem with that.
Please refer to http://www.biad.bcu.ac.uk/research/rti/riadm/issue6/issue%206.pdf
Final Thoughts
The perfect preservation of Jake’s lunch and the Andrée, Strindberg and Fraenkel expedition’s frozen film beautifully illustrates how one or two simple facts can destroy a theory, and how some people “cherry-pick” their data to support their claims.
If Irvine truly lies at rest in the Yellow Band near the same area as the artifacts found at the 1933 high camp, then the camera has experienced the same dry climactic conditions. So there is an excellent chance the camera hasn’t rusted open, and all the “copious” amounts of nefarious water and oxygen molecules at high altitude haven’t done their dirty deed.
Searchers like Tom Holzel, Jochen Hemmleb, and Graham Hoyland understand the chances of the camera film being ruined or not. Researchers have always known that the film is vulnerable because of the brittleness of old cellulose nitrate-base film, or cosmic rays fogging the emulsion.
No one has ever claimed the film will be in pristine condition, and everybody knows that if Irvine is found without the camera or if the film yielded no images, then the tale Irvine’s location and possessions will tell of their last climb will still be a treasure trove of information.
It’s definitely worth a look.
Pete Poston
Philomath, OR
USA
References (accessed 12/15/2011)
- http://www.everest1953.co.uk/MallorysCamera.php
- http://velocitypress.com/mallory_irvine.shtml#A127_Film
- K. J. Edwards1,D. R. Rogers1, C. O. Wirsen, and T. M. McCollom, “Isolation and Characterization of Novel Psychrophilic, Neutrophilic, Fe-Oxidizing, Chemolithoautotrophic α- and γ-Proteobacteria from the Deep Sea”, Applied and Environmental Microbiology, December 2011, Volume 77, Issue 23
- Sabrina Hedrich, Michael Schlomann, and D. Barrie Johnson, “The iron-oxidizing proteobacteria” Microbiology (2011), 157, 1551–1564
- Yongqin Liu, Tandong Yao, Nianzhi Jiao, Shichang Kang, Yonghui Zeng, Sijun Huang, “Microbial community structure in moraine lakes and glacial meltwaters, Mount Everest”. Microbiology Letters, Volume 265, Issue 1, pages 98–105, December 2006
- see statistics for 2002-2004 on ExplorersWeb.com
- http://www.ncdc.noaa.gov/paleo/icecore/trop/rongbuk/rongbuk.html and the data http://www.ncdc.noaa.gov/paleo/icecore/trop/rongbuk/rongbuk_data.html
- “Shackleton's
Biscuits: A Case for Not Eating Artifacts on Mount Everest”
![]()
Appendix – Calculations
I: Water vapor pressure at the summit
relative humidity = actual/max
0.7 = actual/max
Actual = 0.7 x max
Actual = 0.7 x 0.00095 atm = 0.00067 atm H2O
II: Camera dimensions
The camera dimensions are 12.2cm x 4.3cm x 2.4cm which equals a volume of –
126 cm3 = 126 mL or 0.126 L volume camera
III: Volume of water in camera volume
How many grams of H2O are in this volume? Using the Ideal Gas Law -
n = PV/RT where n = moles H2O, P = pressure (atm), R = idea gas law constant (0.0821 Latm/mol K), and T is the absolute Kelvin temperature
n = (0.00067 atm)(0.126 L)/(0.0821 Latm/mol K)(253 K) = 4.1 x 10-6 moles H2O
grams H2O = 4.1 x 10-6 moles x 18.0 g/mole = 7.4 x 10-5 grams or 74 micrograms of H2O in camera
given the density of water is still about 1.0 g/cc at -20 oC, then the volume of water only is 74 microliters of water in the camera
IV: Grams oxygen
How many grams of O2 are in camera volume when the pressure is reduced by a third? Using 20% oxygen in the atmosphere -
0.20 atm/3 = 0.067 atm O2 at the summit
Using the Ideal Gas Law again -
n = (0.067 atm)(0.126 L)/(0.0821 Latm/mol K)(253 K) = 4.1 x 10-4 moles O2
grams O2 = 4.1 x 10-4 moles x 32.0 g/mole = 0.013 grams = 13 milligrams of O2 inside the camera
V: solubility of oxygen in water
With only 74 microliters of water, obviously not all 13 milligrams of oxygen in the camera with dissolve.
The solubility is given by
Henry’s Law - kH varies with temperature so value at 298K used. Recognize
that kH increases with temperature so this calculation gives minimum
concentration – probably about 4X higher
(ref: http://en.wikipedia.org/wiki/Henry's_law)
PO2= kH [O2] the value of kH @25oC = 769 L atm/mol
[O2] = PO2/kH = (0.067 atm)/769 L atm/mol = 8.7 x 10-5 mol/L water solubility O2
Multiply 4X = 3.5 x 10-4 mol/L water solubility
This corresponds to 11 milligrams of O2 per L
VI: Grams oxygen
The volume of just water in the camera calculated in Part III is 74 microliters, so the moles of dissolved oxygen is –
3.5 x 10-4 mol oxygen per L of water x 74 x 10-6 Liter = 2.6 x 10-8 moles dissolved oxygen
the mass of the dissolved oxygen is -
2.6 x 10-8 moles x 32 g/mol = 8.2 x 10-7 grams or about 1.0 microgram of dissolved oxygen
VII: The amount of iron consumed by this amount of water
Recognizing that there are multiple steps - including the formation of iron(III) hydroxide as an intermediate - rusting can be summarized by the following reaction -
4Fe(s) + 3O2(g) ® 2Fe2O3(s)
grams Fe = 2.6 x 10-8 moles O2 x 4 moles Fe/3 moles O2 x 55.8 g Fe/mole =
1.9 x 10-6 grams of Fe or about 2 micrograms Fe consumed (two millionths of a gram)

Articles and Editorials
![]() Harvey V. Lankford, MD, has written a paper documenting the origin of the term "Glacier Lassitude" as a diagnosis for the debilitating effect of altitude as experienced by members of the early British Everest expeditions.
Harvey V. Lankford, MD, has written a paper documenting the origin of the term "Glacier Lassitude" as a diagnosis for the debilitating effect of altitude as experienced by members of the early British Everest expeditions.
![]() My new theory about Mallory and Irvine's last climb, where I believe Odell's sighting was erroneous, and have them taking the Couloir route instead.
My new theory about Mallory and Irvine's last climb, where I believe Odell's sighting was erroneous, and have them taking the Couloir route instead.
Part 1: the ascent
Part 2: the descent
![]() Warwick Pryce is a new researcher who has arrived on the scene, and he has a new theory about how Andrew Irvine could have been the first person to stand on the top of the world.
Warwick Pryce is a new researcher who has arrived on the scene, and he has a new theory about how Andrew Irvine could have been the first person to stand on the top of the world.
Wim Kohsiek has a new interpretation of what Mallory's altimeter can tell us based on scientific applications of meterology.
Mallory and Irvine researcher Wim Kohsiek has two new thought-provoking articles about Mallory's watch and Irvine's location:
Mallory's Watch - Does it Really Point to 12:50 PM?
1924 Oxygen by Richard McQuet and Pete Poston
Why the Camera and Film are not Doomed to Destruction!
The Politics of Mallory and Irvine
Why Andrew Irvine Will Not be Found in a Sleeping Bag! Part 1 and Part 2 on ExplorersWeb
Chomolungma Nirvana: The Routes of Mount Everest
Rust Marks on Mallory's Altimeter
Mystery of Mallory and Irvine's Fate Google Earth Tour - my own ideas in 3-D with audio!
Little Known Free-Solo Ascent of the Second Step in 2001 by Theo Fritsche - I should never have written this - Anker and Houlding deserve credit for the first free ascent
Criticisms of the 2004 EverestNews.com search for Irvine --
The Mystery of Mallory and Irvine's Fate (with J. Hemmleb): Part 1, Part 2, Part 3, Part 4, Part 5.
Mallory and Irvine - Comments on the 'real Second Step' route: Part 1 and Part 2
Conrad Anker's comments on the unlikeliness of a direct route up the prow of the 2nd Step
Articles about my heroes Walter Bonatti and Chris Bonington --
Spilling the Beans - Lino Lacedelli's Book "Price of Conquest: Confessions from the First Ascent of K2" Part 1 and Part 2
The Life and Climbs of Chris Bonington, Part 1, Part 2, Part 3, Part 4, Part 5 final - interview
About Me
 Celebrating my 50th birthday on pitch 3 of Prodigal Son, Zion National Park, Utah
Celebrating my 50th birthday on pitch 3 of Prodigal Son, Zion National Park, Utah
In my free time, I love to photograph and hike the spectacular redrock wilderness of the Colorado Plateau - please visit my Colorado Plateau Homepage.
And for most of my life I've been fascinated with the history, people, and culture of the Himalayas and Karakoram - browse my Mount Everest Trek (1996), Overland Journey from Kathmandu to Lhasa (2000), and K2 Base Camp Trek (2007) webpages.
As for my employment, I work for Western Oregon University where I have been a Professor of Chemistry for the last 20 years. My research interests are in applications of Laser Raman Spectroscopy to such diverse fields as Nanotechnology, Analytical Chemistry, and even a bit of Achaeology through the study of rock art pigments found in the Colorado Plateau. You can access my academic webpage here.

|
Copyright (c) 2004-2022 Pete Poston. All rights reserved. Visitor's Agreement |




